Background: Traditional “3+7” intense chemotherapy (IC) regimen is the standard treatment backbone for fitness patients with newly diagnosed AML. The azacitidine plus venetoclax (AB) regimen has recently been recommended for elderly AML patients or those ineligible for IC treatment. However, neither the “3+7” IC nor “AB” regimen unmet the needs ofadequate safety and effectiveness, especially on aspects of early death and some subtypes of leukemia, such as M5, or with RUNX1, FLT3-ITD, TP53 mutation, or with MCL-1 up-expression. Chidamide, a novel oral histone deacetylase inhibitor, can overcome the upregulating of MCL-1 expression induced by venetoclax, and synergy with azacitidine and venetoclax induces apoptosis of acute myeloid leukemia cells. Azacitidine-Venetoclax -Chidamide (ABC) combination regimen had been successfully used for refractory relapsed AML and newly diagnosed AML-M5 by Prof. Bin Xu and Prof. Shengli Xue, respectively. Whether the ABC regimen can be comparable to the “3+7” IC regimen in newly diagnosed AML induction therapy is still unknown.
Aim: This pilot study aims to evaluate the efficacy and safety of the ABC regimen versus the “3+7” IC regimen in newly diagnosed AML.
Method: A total of 112 patients with newly diagnosed AML (not APL) were enrolled in this study. Thirty-one patients from the ABC Collaboration Group were unfitness or unwilling to undergo IC therapy and received ABC regimen treatment from May 2022 to July 2023. Eighty-one patients from GDPH in the AML Collaboration Group of South China were treated with a “3+7” (IC) regimen between November 2010 and August 2013.
Treatment protocol: The ABC-28d protocol consists of azacitidine 75mg/M 2, d1-7; venetoclax 100mg d1, 200mg d2, 400mg d3-28, and Chidamide 10mg, d1-6/week for 2 weeks, 28 days as one cycle. The ABC-14d regimen consists of azacitidine 75mg/M 2, d1-7; venetoclax 100mg d1, 200mg d2, 400mg d3-14, and Chidamide 5mg, d1-6/week for 2 weeks, 28 days as one cycle. “3+7” IC regimen consists of idarubicin 10mg/M 2, d1-3; cytarabine 100 mg/M 2, d1-7.
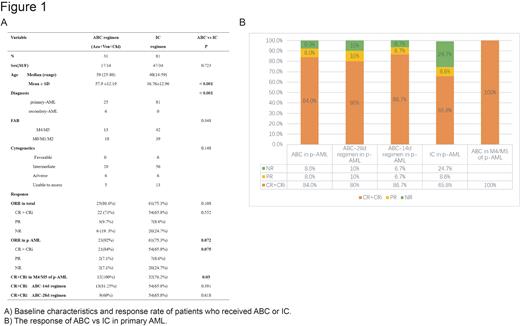
Baseline characteristics for patients are listed in Table 1. We compared the complete response (CR) and overall response rate (ORR= CR+ CR with incomplete recovery of blood counts CRi + partial response PR) after the first course of treatment, cumulative response rate, and adverse events between these two cohorts.
Results: The outcome data of the ABC regimen was updated as of July 2023 for a median follow-up of 78 days (range 28- 402) for the ABC cohort. The ORR and CR/CRi rate of the ABC cohort after the first cycle therapy was not significantly different from the IC cohort (80.6% vs 75.3%, P =0.108 and 71% vs 65.8%, P=0.552). And the cumulative response rate of the ABC cohort was 87.1%.
In primary AML, the CR rate of patients in the ABC cohort was higher than the IC cohort (84% vs 65.8%, P=0.075). Of concern, all 12 patients with M4/M5 treated with the ABC regimen achieved CR (100% vs 76.2%, P=0.03). In secondary AML, the ABC regimen was also poor (CR/CRi rate 33.3%). The ABC regimen is not only applicable to the elder patients (CR rate 100%, 15/15) but also young patients(<60 Y) same as the IC regimen ( CR rate: 71.4% vs 65.8%, P=0.726). The response was summarized in Table 1 and Figure 1.
Up to 30 July 2023, the ABC cohort has not reached the median remission duration and overall survival (OS). Grade 3 or 4 hematologic adverse events occurred in 81.2% of patients. Neutrophil and platelet median recovery times were 21 days(0-58) and 16 days (0-58), respectively. Patients in the ABC-14d cohort had lower rates of hematologic AEs than ABC-28d cohort (Neutrophil: 12.13±14.33 vs 26.33±16.89, P =0.024 and Platelet: 11.88±13.92 vs 24.58±18.59, P = 0.048). The most frequent non-hematologic AEs of any grade were neutropenic fever (n=15), pneumonia (n=8) and reversible nephrotoxicity (n=4). In the ABC cohort, no patient experienced early death at week 4 or 8.
Conclusions: This preliminary study showed that the safety and efficacy of the ABC regimen were comparable to the traditional “3+7” regimen for newly diagnosed AML, even better for the subtype of AML-M4/M5. The ABC-14 days regimen showed comparable efficacy but better tolerability. However, whether the ABC regimen can replace the traditional intense chemotherapy needs to be confirmed by further randomized controlled trials.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal